Title: Climate change is caused by the absorption of energy ultraviolet by oxygen.
Author; Rogelio Pérez C
Summary;
The problems of climate change occur primarily by an increase in the temperature of the planet. The Science teaches that the heat in the earth originates mainly after the solar constant, by a greenhouse effect caused by greenhouse gases that absorb infrared energy (heat), therefore the problem of global warming, is described as an increase in the greenhouse effect, caused by the increase of greenhouse gases, because of human activity (anthropogenic), pointing mainly to fossil fuels such as Greatest threat facing the planet. But the amount of atmospheric gases, which absorbs the infrared energy or heat, is only 0.04%, and 99.9% of the atmosphere does not absorb heat, so we can conclude that this percentage is not significant, to originate enough heat in the atmosphere of the Planet. In contrast, oxygen (21%) of atmospheric gases, by absorbing ultraviolet energy, increases the speed of its molecules, increasing the temperature in the atmosphere of the planet, causing heat, and oxygen is a highly reactive gas, which is the only gas in the atmosphere, which by physicochemical processes (ionization) originates light and heat in the upper layers of the atmosphere.
Figure 1. The ionization of oxygen by UV.
In the world is under the influence of global warming, which we also understand under the concept of climate change. The problem of climate change is mainly presented by an increase in the temperature of the planet. The temperature of the planet can be understood mainly as the average velocity of the molecules of the atmosphere, by the absorption of the energies emanated by the sun or another source. Then we understand that an increase in the speed of the molecules, by an increase in the absorption of energy, or the increase of the molecules that cause the heat in the atmosphere, could lead to an increase in the temperature of the planet, and to originate the global warming. Science teaches us that the average temperature of the planet after the solar constant would be -27 º C, but thanks to the gases that absorb heat and reemit it to the earth, the average temperature of the planet is at 15 º C, this process describes it as a greenhouse effect , and global warming describes it as an increase in the greenhouse effect, because of the increase in gases that cause this effect, due to human activity, and points to fossil fuels as the main threat to the planet.
New Vision of the problem.
When we observe, that 99.9% of the molecule of the atmosphere, do not absorb the infrared (heat), then we understand that the so-called greenhouse effect, is not effective to heat the atmosphere, because heat absorbing gases are only 0.04% of all The Molecules of the atmosphere. Under this equivalence we understand that this percentage of gases, not only, is not significant to sustain the heat of the planet, but it hardly originated a global warming.
This paper presents the following solution; Oxygen (21%) of atmospheric gases, by absorbing ultraviolet energy, increases the speed of its molecules, increasing the temperature in the atmosphere of the planet, causing heat, in addition oxygen is a highly reactive gas, which is the only gas in The atmosphere, which by physicochemical processes (ionization) originates light and heat in the upper layers of the atmosphere.
How does oxygen originate heat in the air?, oxygen originates heat directly when it is ionized, because oxygen becomes a conductor of electricity from the air, therefore from heat, the Joule effect explains this, as an irreversible phenomenon through the Which, If a conductor circulates electric current, part of the kinetic energy of the electrons is transformed into heat, which happens with the electricity of the air, that is why in the mid-day there is much more electricity in the air, therefore it makes more heat , which in the morning or at night, where the electricity of the air is less. As the highest amount of oxygen is in the lower layer of the atmosphere, known as the troposphere, where ultraviolet energy is also present in all wavelengths, although in a lesser percentage the higher energy level. Conclude That the absorption of the ultraviolet energy by oxygen, in the lower layer of the atmosphere (troposphere), is the main cause of the heat on earth, because it ionizes the air, converting the atmosphere, into an electric conductor, and therefore heat.
After understanding a new way to originate the heat on the planet, we could explain global warming in two ways, the first by a to increase the oxygen molecules that absorb more ultraviolet energy, which is not happening, and the second as the increase of the amount of ultraviolet energy that reaches the troposphere, which is the approach of this work.
This increased amount of ultraviolet energy is absorbed by the oxygen, which increases the physicochemical process (ionization), resulting in an increase in the atmospheric electricity, therefore, increase of heat.
Formulation of the theory and the definitions.
For more than 100 years scientists know the importance of sun energy in the heat of the air. First to predict global warming: The Nobel Prize Svante Arrhenius, in the 1896 document shows us a context in his writings, where scientists agreed that the air retains the heat of light, and that the increased heat retention of the air is the energy that and they called it Dark (ultraviolet). The concept of the greenhouse effect began to appear at this time, as a retention of both infrared and ultraviolet radiation, they called it, dark light, giving greater importance to the percentage of these gases in the atmosphere.
At the time of 1896 was not known with accuracy the percentage level of gases in the atmosphere, as now, by which favored some by the vapors from the air, and others by the carbonic acid, called carbon dioxide, because they were the gases in the atmosphere that absorbed heat
The work of Nobel Prize proposed an increase in the temperature of 8ºC and 9ºC for Arctic areas if the quantity of CO2, increased in 2-3 times its value[1] , we know today that the amount of CO2 emitted in the Arctic is ten times more than what they have estimated[2] , the CO2, increased in the last 100 years, in 105 ppm in the whole planet, increasing only a 0.8ºC in the average temperature of the planet [3].
Now that we know more specifically the components of the atmosphere and its absorption of the radiation from the sun, we cannot continue with ideas from 120 years ago, where the data that had of the temperatures of the full moon, helped to impose the thesis, of the heating by the Carbonic acid, eliminating the importance of ultraviolet radiation and other theses that existed at the time.
Global warming is one of the problems that affront humans currently. Temperatures are rising several years ago, and the main scientific explanation has generated division among scientists.
The scientific consensus accepts global warming, but I believe that the percentage that does not accept the main explanation for this phenomenon is high, besides that the explanation about global warming, on the part of the scientific community is quite exotic, what Which generates, that some who had doubts about global warming, have begun to reject it. and others, who express doubts about the assumption of a greenhouse effect, are rejected, originating in this way, Not a better understanding of the problem, but a shortage of new scenarios for climate problems.
The scientific hypothesis shows the human activity (anthropogenic), is the main cause of global warming, by the production of the gases that absorb the heat (greenhouse gases), being one of the main dioxides carbon. The explanation of global warming said; it is a consequence of the increase in the greenhouse effect, caused by the increase of the gases that absorb the heat, Up to here, according to the paradigm established is logical, because in the planet according to the science the heat occurs indirectly by a greenhouse effect, caused mainly by the CO2, but all greenhouses known are made of materials solids such as glass or plastic, but the greenhouse also needed a gas within it absorbs the heat, Therefore the explanation of a greenhouse caused by gases, the similarity with the facts with solid material as the glass is very difficult, the scientific hypothesis begins to fail because only 0.04% of the atmosphere are greenhouse gases, and 99.9% of the gases in the atmosphere does not absorb the heat, it can be explained in this way, 0.04% of the gases enclose as a greenhouse at 99.9% of the atmospheric gases, which is very difficult, but if it does, the gases enclosed in this case 99.9%, do not absorb the heat of the greenhouse.
In addition, scientific experiments on how it works the carbon dioxide, to cause the greenhouse effect, are always made with a greenhouse made of glass or plastic. This causes a greater doubt about global warming, due to the greenhouse effect on the planet is caused by gases.
Figure 1. Greenhouse Effect.
Figure 1. Greenhouse Effect.
The atmospheric electricity.
Is the diurnal variation of the network to electromagnetic the atmosphere (or, more general, any electrical system in the atmosphere of a planet) The surface of the Earth, the ionosphere and the atmosphere is known as the "electrical circuit global atmospheric". The atmospheric electricity is a cross-curricular theme. There is always free electricity in the air and in the clouds, which act by induction on earth and electromagnetic devices[4]. Experiments have shown that there is always free electricity in the atmosphere, which is sometimes negative and other times positive, but most of the time is generally positive, and the intensity of this free electricity is greater at noon than in the morning or n Ocher and is greater in winter than in summer.[5]
Ionization
Is the chemical or physical phenomenon by which ions are produced, these are atoms or molecules electrically charged due to the excess or lack of electrons with respect to an atom or neutral molecule[6].
The atmosphere
Is the layer of gas that surrounds a cosmic body, the gases are attracted by the gravity of the body and are kept in him if the severity is sufficient and the temperature of the atmosphere is low, some planets are formed mainly by gas, by what they have atmospheres very deep.
Atmospheres of other planets
The planet Mercury that is closer to the sun, the carbon dioxide is equivalent to 3.6% of its atmosphere, by day the temperature can reach (427 °c), at night when the temperature can reach (-170 °c)[7], but its atmosphere is very dim, it does not retain the heat , on Earth it seems the opposite, the dim layer of the atmosphere known as the ionosphere, can reach 1500ºC, or more.
On the planet Venus, carbon dioxide is 96%, it is the hottest planet in the solar system above the mercury, which is closer to the sun, its maximum temperature is (499°C), the average is (463°C), the minimum is (-45ºC)[8]. But the higher temperature of the planet Venus, is overcome by almost three times for a layer tenuous atmosphere of the earth, with a 0.1% of the gases, known as the ionosphere, which shows that the gases that absorb energy Ionizing, on the atmosphere, as the gas of oxygen, is the most effective way to lead to high temperatures in a planet, that greenhouse gases. Because the temperature of the ionosphere on earth can overcome the 1500ºC.[9]
In the atmosphere of the planet Mars, the carbon dioxide is equivalent to 95.3, and its average temperature is (-46°C)[10] , but we also have to say that receives less rays of sunlight that the earth and its atmosphere is less dense than the Earth, although it is paradoxical that the layer of the atmosphere of the earth (ionosphere), which is less dense than the Mars, and with less CO2, is more hot.
The earth has the hottest atmosphere in the solar system.
When we look up into the Earth's atmosphere, to the ionosphere, we can see that we have the hottest atmosphere of the solar system, with just 0.1% of the mass of the atmosphere, this tenuous layer of gas makes the Earth's atmosphere , the hottest solar system, 3 times hotter than Mercury and Venus, which are the planets closest to the Sun, the ionosphere has a maximum temperature of 1500°C.
Figure 3. Temperature of ionosphere.
What gas in the atmosphere produces these high temperatures in the ionosphere?
We know that in the atmosphere the main gases are; Nitrogen 78%, oxygen 21%, argon 0.9% and carbon dioxide 0.04%, but the only highly reactive gas, which absorbs ionizing energy like ultraviolet, is oxygen, so we can conclude; That the ionization of oxygen is the cause of these high temperatures in the ionosphere.
The composition of the Earth's atmosphere.
The atmosphere of the planet is made up of 78% of nitrogen which is an inert gas that usually does not react with other substances. 21% oxygen, which is a highly reactive gas. 9% argon, and 01 percent of other gases.
Almost the whole of the air (95%) has less than 30 km high, being more than 75% in the troposphere. The air form in the troposphere a mixture of gases homogeneous to the point that their behavior is equivalent to that which would have if it were composed of a single gas.
• Nitrogen: constitutes 78% of the volume of air. It is composed of molecules that have two nitrogen atoms, so that your formula is n2. It is an inert gas, i.e. that usually does not react with other substances.
• Oxygen: represents the 21% of the volume of air. It is formed by molecules of two atoms of oxygen and its formula is O2. It is a gas very reactive and most of the living beings need to live.
• Argon: contributes in 0.9% of the volume of air. It is a noble gas that does not react with any substance.
• Carbon dioxide: it is composed of molecules of a carbon atom and two oxygen atoms, so that your formula is CO2. Represents the 0.03 per cent of the volume of air and participates in the biological processes and climatically very important. The plant needs to carry out photosynthesis and is the residue of the breathing and combustion reactions that occur, for example, in a forest fire or in the engine of a car.
This gas helps retain mainly the heat of radiation terrestrial and atmospheric, so that is the main cause of the greenhouse effect.
• Ozone: It is a minority of gases found in the stratosphere. Its formula is O3, because its molecules have three oxygen atoms. It is of great importance for life on our planet because its production of atmospheric oxygen absorbs most of the ultraviolet rays of the sun.
• Water vapor: It is in very variable quantity and participates in the formation of clouds or fog. It is one of the gases that causes the greenhouse effect.
• Solid Particles and liquid: in the air there are many solid particles in suspension, like, for example, dust that raises the wind or the pollen. These materials have a very variable distribution, depending on the wind and the human activity. Between the liquids, the most important substance is the water in suspension that is located in the clouds [iv]
Origin atmospheric heat .
The solar radiation is the main energy source and virtually the only for the atmosphere of our planet. This solar radiation reaches us in the form of heatstroke: rays of light and heat of different wavelengths that constitute the visible spectrum (luminous rays) and the shorter wavelength not visible (UV) and longer wavelength (infrared rays, which are also not visible).
Thus, the visible spectrum is in the middle of the spectrum formed by the solar radiation that reaches our planet, and more specifically to the terrestrial atmosphere.
The solar radiation passes through the atmosphere without heating it, because the air is diathermanous it can pass through the solar rays without heating. But this solar radiation, when it reaches the terrestrial or maritime surface, is transformed by increasing its wavelength and can heat the water as well as the soil and the lower layers of the air. Thus, this warming of the Earth's atmosphere is not direct but indirect from infrared rays of greater wavelength that are re-issued by the land surface warm.2
The heating in the lower layers of air is due to two closely related phenomena:
1. The highest atmospheric pressure of the air at low altitude. This is derived from the fact that the air is compressible, i.e. it can be compressed by its own weight. and low-altitude compressed air can absorb much more heat than expanded air that is at high levels.
2. The low range of waves reflected by the Earth's surface: These waves are of infrared radiation (long wave) and lose their thermal energy very quickly after being emitted.
Global warming and climate change refers to the observed increase in more than a century of the temperature of the climate system of the Earth and its effects,[11] Multiple lines of scientific evidence show that the climate system is warming up,[12] Many of the changes observed since the 1950s have no precedent in the instrumental record temperatures that extends to the middle of the nineteenth century or in the proxy logs paleo climate that cover thousands of years.[13]
Greenhouse effect
Thanks to the atmosphere, the earth has no great thermal contrasts; Due to the natural greenhouse effect, which is produced by all gaseous components of the air, which absorb much of the infrared radiation re-emitted by the terrestrial surface; This heat is retained in the atmosphere instead of being lost in space thanks to two physical characteristics of the air; Its compressibility, which compresses the air in contact with the Earth's surface by the very weight of the atmosphere, which, in turn, determines the greater heat absorption of the air subjected to greater pressure and the diathermancy, which means that the atmosphere allows the Solar radiation almost without heating (direct absorption of heat from solar rays is very scarce),While that absorbs a large amount of heat dark, forwarded by the land surface and, above all, aquatic of our planet. This greenhouse effect has a key role in the smooth average temperatures of the planet. Thus, taking into account the solar constant (calories that reach the surface of the land per square centimeter and per minute), the average temperature of the earth would be -27°C, incompatible with life as we know it; In contrast, its real value is approximately 15°C due precisely to the greenhouse effect.[14]
Ultraviolet radiation
Is called ultraviolet radiation or UV radiation to the electromagnetic radiation whose wavelength is covered approximately between 400 nm (4x10-7 m) and 15 Nm (1.5x10-8 m).
The greater part of the ultraviolet radiation that reaches the earth occurs in the forms UV-C, UV-B and UV-A; mainly in the latter, due to absorption by the atmosphere. These ranges are related with the damage they produce in the human being:
The UV-C (the most harmful for life) does not reach the earth to be absorbed by the oxygen and ozone in the atmosphere; the UV-B radiation is partially absorbed by the ozone and only reaches the surface of the earth in a minimum percentage, despite that can cause damage to the skin.[15]
Increased ultraviolet radiation
NASA scientists who analyzed 30 years of satellite data found that the amount of ultraviolet (UV) radiation that reaches the surface of the land has increased significantly in the last three decades. Most of the increase has occurred in the middle and high latitudes, and there has been little or no increase in tropical regions.[16]
The researchers speculate that this increase in the flow of ultraviolet light may have been caused by the depletion of the ozone layer, as a result of the increase of aerosols due to the seasonal storms and fires in the area. In addition, there was a large solar flare just two weeks before it is recorded flows UV higher.
Although the evidence that relates the event solar with radiation record is only circumstantial, it is known that the particles of these eruptions affect atmospheric chemistry and can increase the ozone depletion.[17]
The investigation recently published for the first time examines changes in ultraviolet radiation (UV) in Australia for a period of fifty years (1959-2009). The investigation found that there has been a total annual increase in UV levels from 2 per cent to 6 per cent since the 1990s, to locations throughout Australia.[18]
Prognosis Clear Sky UV Index
The Index Clear Sky UV is a measure for the UV radiation effective (1 unit equals 25 MW / m2) that reaches the surface of the Earth. The UV index of the cloudless sky is based on the spectrum of action of CIE for the susceptibility of the skin Caucasian to sunburn (erythema) and is valid for conditions free of clouds in the solar noon local, i.e., when the sun is highest in the sky. The forecasts are provided for today and the next few days.
Figure 4. prognosis. UV. index
Oxygen
Is a chemical element of atomic number 8 and represented by the symbol O, under normal pressure and temperature conditions, oxygen is a colorless and odorless gas with O2 molecular formula, in which two oxygen atoms are linked to the electronics of Triple-State configuration. This link has a two-link order and is generally simplified in the descriptions as a double link or as a combination of a two-electron link and two three-electron links.
The oxygen is a very reactive than form oxides with all elements.
Triplet oxygen-which should not be confused with ozone, O3-is the fundamental state of the O2 molecule, which has two unpaired electrons occupying two generated molecular orbits. This orbital is classified as anti-binders, weaken the binding order of three to two, so the dioxygen bond is weaker than the triple bond of diatomic nitrogen, in which all the orbital bonds of the molecular bonds are filled, but some Anti-orbital binding is not.
In its normal triplet form, the O2 molecules are paramagnetic; In other words, in the presence of a magnetic field, they form a magnet, due to the magnetic momentum of the rotation of the decoupled electrons in the molecule and the interaction of the negative exchange between the contiguous O2 molecules.[19]
Creation of ozone
Ozone occurs naturally in the stratosphere when radiation High Energy Solar hits the oxygen molecules, O2, and makes the two oxygen atoms are separated in a process called photolysis[20]
As it increases, the temperature in the stratosphere increases. This rise in temperature is because ultraviolet rays transformed oxygen into ozone, a process involving heat: ionized air becomes a good conductor of electricity and therefore the heat
It is known as Joule effect to the irreversible phenomenon by means of which, if a conductor circulates electric current, part of the kinetic energy of the electrons is transformed into heat. Because of the shocks that it suffers with the atoms of the conductive material by which they circulate raising the temperature of the same one.
Figure 5. Ozone formation.
It is called the electromagnetic spectrum to the power distribution of the set of electromagnetic waves. Refer to an object is called the electromagnetic spectrum or simply spectrum to the electromagnetic radiation emitted (emission spectrum) or absorbed (absorption spectrum) to a substance.[21]
Figure 6. Absorption of Oxygen.
Is a model that describes the three elements necessary to generate most of fires: a fuel, an oxidant (an oxidizing agent such as oxygen) and the activation energy. When these factors are combined in the correct proportion, the fire is triggered. On the other hand, it is also possible to prevent or attack a fire by eliminating one of them.[22]
Without enough heat, the fire cannot start or spread. It can be eliminated by introducing a compound that takes a part of the heat available for the reaction. Water is generally used, which takes the energy to move to the gaseous state. They are also effective powders or gases with the same function.
Without the fuel, the fire stops. It can be eliminated naturally, consumed by flames, or artificially, through chemical and physical processes that prevent fire from accessing fuel. This aspect is very significant in the fire extinguishing (for example, through the firewall, as well as in the controlled fire).
The lack of oxygen prevents the fire from beginning and spreading.
Figure 7. fire triangle.
On Earth is needed a minimum of 14% oxygen in the atmosphere to originate a flame or fire.
Increasing the oxygen percentage in the air from its initial 21% significantly increases the temperature of the flame reached with any fuel. For example, the natural gas burned in the air reaches a temperature of 1938 °c, whereas if it burns in an environment with 23% of O2 reaches 2004°C[23], therefore we can conclude that the oxygen level increases the temperature of the flame or light.
In the industry oxygen as a source of heat.
Oxy-combustion; it is a technique that consists in separating the nitrogen of the atmosphere of an oven, and replace it with pure oxygen, which increases the temperature. When the temperature is 3000°C by the injection of pure oxygen, then is recirculated the CO2 produced by the oven to reduce the temperature, which lowers the temperature of the oven until the1900°C[24].
Presentation of data and results.
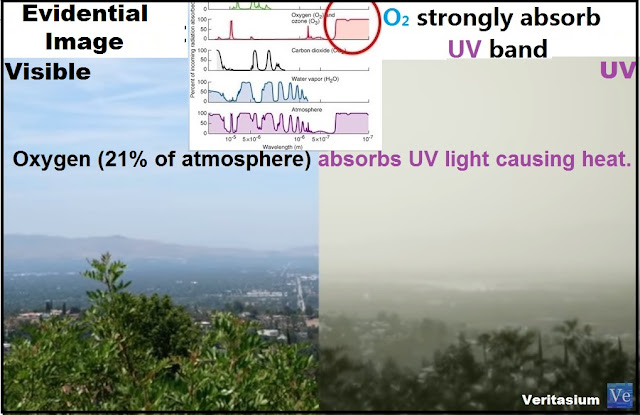
The oxygen is the second gas more abundant in the atmosphere, equivalent to 21% of all atmospheric gases, is the only gas in the atmosphere highly reactive, which when combined with other elements produces heat and light, in the upper layers of the atmosphere we see to oxygen producing high temperature, and light. It is the product of oxygen ionization.
This heat generation process occurs when oxygen molecules absorb ultraviolet energy, ultraviolet energy increases the speed of molecules and ionizing them, the oxygen causes heat directly when is ionized, because oxygen becomes a conductor of electricity in the air, therefore heat, the Joule effect explains this, as an irreversible phenomenon through which, if a driver circulates electric current, part of the kinetic energy of the electrons is transformed into heat, which happens with the ionization of oxygen, Which cause the electricity in the air and therefore heat, it is for this reason that in the middle of the day there is much more electricity in the air, therefore, makes more heat, which in the morning or in the evening where the electricity in the air is less.
We can see this in the ionosphere, where the atmosphere absorbs a lot of energy and its molecules are ionized. Reaching the 1500°C, but the heat transmitted is small, because the mass of air of this layer is very thin around 0.1%. The absorption of ultraviolet radiation by the oxygen occurs from the top of the atmosphere, to the lowest in the troposphere, where is located the largest amount of air in the atmosphere.
Ultraviolet can be divided into three types of rays.
UV A, long wave is those that most reach the troposphere and stimulate the oxygen molecule to increase the temperature.
UV B, short-wave, very damaging that can cause burns, is absorbed by ozone. Increase the temperature from-60 °c to 0 °c, but also reach the troposphere and produce skin cancer, and heat by ionizing the oxygen.
UV C, shorter wave and greater danger, is absorbed by oxygen in the upper part of the atmosphere, originating light. A small part reaches the oceans, but before reaching the water, it has to go through the oxygen on the surface of the water.
Science teaches us that; The solar radiation passes through the atmosphere without heating it, because the air is diathermanous it is allowed to pass through the solar rays without heating. But this solar radiation, when it reaches the terrestrial or maritime surface, is transformed by increasing its wavelength and can heat the water as well as the soil and the lower layers of the air. Thus, this warming of the Earth's atmosphere is not direct but indirect from the higher wavelength infrared rays that are re-emitted by the hot earth surface.
When we see that 99.9% of atmospheric gases do not absorb infrared energy directly, or indirectly, and only absorb this energy 0.04%, of all gases in the atmosphere, we can reach the conclusion; That infrared energy, is not the energy that heats the atmosphere as science tells us, therefore, it does not originate global warming,
Global warming can be explained by an increase in gases, or by an increase in electromagnetic energy. In this case it is the ultraviolet energy, which is known to have increased in the last 50 years. In addition we must not forget the agreements climate, for the recovery of the ozone layer, and although the ozone layer has recovered, we cannot ignore, that for there to be a recovery of the ozone layer, is required primarily, that has greater ionization of oxygen, because the product of this process physicochemical between the oxygen and the ultraviolet energy, originates the ozone layer in the atmosphere, which then help to a better filter from the ultraviolet energy, With a chemical composition of three oxygen atoms.
According to the projections in places where ultraviolet radiation is much more intense than in the equator, warming in these areas is much more intense than in other parts of the planet.
NASA scientists found that in tropical regions where ultraviolet radiation is more intense, this has not increased, which can be explained by a greater absorption of this ultraviolet radiation by oxygen in these areas, which is transformed into an Increase in temperature in these areas of the planet.
Figure 8. Relationship between the ultraviolet and Global warming.
Science has taught us that there is a correlation between the amount of CO2 in the atmosphere and the temperature of the planet, but when we review the same historical data, we realize that this is not true, and the same science does not dare to say, How much CO2 is needs to increase in a degree the average temperature of the planet, the work of more than 100 years ago, with which established the paradigm, established the projections of temperature by increase of CO2, but which are not in line with the current reality, example; in 1880 had 294ppm CO2 in the atmosphere and the average temperature was 14ºC, you might think that with 7.1ppm, would increase the temperature by one degree, but 100 years later, we see that an increase of 105ppm, the average temperature increased only in 0.8ºC.
Let us look at this table of proportion of CO2 and temperature per month;
According to this table the coldest month of the year 2015, was one of the months of higher level of CO2 in the atmosphere, and the levels of CO2 between these months do not agree with the correlation of the temperature.
Figure 9. Comparison between the CO2 and temperature per month.
According to the 10 hottest years recorded since 1998, it is very difficult to demonstrate a relationship between the CO2 in the atmosphere and global warming. Because the years that show greater increases in temperatures that exceed to other years, the same is not true with the average CO2 in the atmosphere in those years.
Figure 10. Comparison between the CO2 and temperature per year.
Variation of temperature anomaly in CO2 310-311ppm, between 1936 and 950.
The table shows a maximum variation of 0.44ºC of anomaly annual of temperatures, when the annual average of CO2 in the atmosphere, did not ranged significantly, and remained in 310ppm and 311ppm. between 1936 and 1950.
The temperature anomaly variation between 1936 and 1950, it begins with an increase of 0.39ºC on1936 (-0.11 º C), and on 1944 (0.28ºC), then decreases 0.44ºC, betwenn1944 (0.28 º C) and 1950 (-0.16 º C), which is important, because the anomaly of the annual temperature, in the last 100 years was increase at 0.8ºC.
The anomaly of 1944 of the temperature was 0.28ºC, came with 310.2ppm CO2 in the Atmosphere, in 1993 with 357.1ppm CO2 in the atmosphere, also had an abnormality of 0.28ºC.
Variation of the anomaly of the average annual temperature vs the annual amount of CO2 in the atmosphere of 294ppm. Between 1887-1897. The table shows a maximum variation of the annual temperature anomaly of-0.24 º C, when the amount of CO2, not change in a decade, and remained at 294 ppm. Between the years 1887 and 1897.
See this level of variation of the anomaly of the temperature in the same amount of CO2 in the atmosphere, is important because in the past 100 years, only 0.8ºC increase the variation of the anomaly of the temperature, in an increase of 105 ppm of CO2 in the atmosphere of 1880(294ppm CO2) until 2015(399ppm CO2).
What would be the percentage of heat that would add the greenhouse gases, and the oxygen in the atmosphere?
The Oxygen absorbs the ultraviolet to cause heat and that is equivalent to 20.94% of the atmosphere and greenhouse gases are equivalent to 0.04%, then the 99.8 per cent of the heat in the earth is caused by the oxygen, and 0.2% is the quantity of heat that reemitted the greenhouse gases in the earth.
Table 3. Percentage of the gases responsible for the heat.
Taking into account the solar constant (calories reaching the surface of the Earth per square centimeter and per minute), the average temperature of the planet would be -27 ° C, incompatible with life as we know it; instead, its real value is about 15°C due precisely to the greenhouse effect[25].
The amount of CO2 in the Earth's atmosphere is 400ppm (0.04%) and the average temperature is 15 °C.
The amount of CO2 in the Earth's atmosphere in 1880 was 294ppm (0.03%) and the average temperature was 14ºC. After the solar constant the planet would be in -27ºC, but by the greenhouse effect was at 14ºC. A contribution of 41ºC.
294ppm/41°C=7.1 ppm. In theory 100 years ago, the concentration of 7.1 ppm of CO2 in the atmosphere was necessary to get 1°C of the greenhouse effect.
From 1880 (CO2 294ppm) to 2015 (CO2 399ppm) in an increase 105ppm of CO2 in the atmosphere, the average temperature of the Earth's surface has risen approximately 1.4ºF (0.8 °C.)[26]
In practice is needed in the atmosphere of the earth 131.2ppm CO2, to achieve 1°C. (105ppm/0.8°C =131.2pmm)
The planet Venus being closer to the sun, to reach an average temperature of 463°C, needs a 96.5% CO2 in its atmosphere.
In theory, the Earth to reach the same average temperature of the planet Venus, would only need 0.33% of CO2 in its atmosphere.
(294ppm/41ºC = 7.1 ppm x463ºC= 3.320 ppm/10000=0.33%)
In practice, the earth would only need 6% CO2 in its atmosphere, to reach the same average temperature as Venus.
(105ppm/0.8°C =131.2ppmX463°C=60.768.7ppm/10000=6%)
The greenhouse effect by CO2, cannot explain the average temperature of the Earth
Atmosphere of Venus is 90 times denser than that on Earth and it is made of 96.5% of CO2 and a 3% of nitrogen. This means that both planets have the same amount of Nitrogen on their atmospheres. Surprisingly the CO2 on Earth is stored on calcite type rocks and if we would convert the CO2 on these rocks into atmospheric CO2 it would amount to the same amount of CO2 that there is on Venus' atmosphere.[27]
If planet Earth and Venus have the same amount of nitrogen, then we can say that both have 780.900 ppm of nitrogen in their atmospheres, because that is the amount that is in the atmosphere of the Earth, which is equivalent to 78% of the Earth's atmosphere[28] and 3% of the atmosphere of Venus.
Then, 78% of nitrogen in the Earth's atmosphere, and 3% of nitrogen in Venus, is equivalent to 780.900 ppm[29].
The 1% of the atmosphere on Venus is equal to (780.900ppm/3=260.300ppm) 260.300ppm.
(96.5 x 260.300 = 24.884.680) 96.5% of Venus's atmosphere is equivalent to 24,884,680 or 24 times ppm
24,884,680 or 24 times ppm is the 96.5% CO2 in the atmosphere of Venus, and 400ppm is the 0.04% of the atmosphere on Earth. `
(24.884.680/400 = 62.211) The amount of CO2 on Venus, is 62,211 times more than on Earth.
(41 x 62.211 = 2.550.651) If with the 400ppm of CO2 on Earth, there is an average temperature of 14 °C (41ºC by the greenhouse effect on Earth), with 24.884.680 or 24 times ppm could have an average temperature in the earth of 2.550.651°C.
The 96.5% CO2 in the atmosphere of Venus is roughly 24,884,680 or 24 times ppm, that's 2.488.6% on Earth. In other words, almost 25 times the Earth's atmospheric mass.
Venus being closer to the sun, needs 24.884.680 or 24 times ppm of CO2 in its atmosphere, to have 463ºC of average temperature.
We are taught that human activity, increases the amount of CO2 in the atmosphere, the big industries are blamed, but NASA data, show us that countries not developed with fewer people, throw more CO2 in the atmosphere at certain times of the year , due to the decomposition of the organic material of the great vegetations that these countries have, especially in warmer seasons, That is why in these stations, we see that the underdeveloped countries launch more CO2 to the atmosphere that the industrialized countries.
Finally, the tables of absorption of electromagnetic radiation show us that the great gases in the atmosphere, the only that absorbs radiation from the sun directly and indirectly is the oxygen, which absorbs almost all the band of the ultraviolet wavelength, in comparison to the absorption of infrared energy by greenhouse gases.
Figure 12. absorption of solar radiation

Conclusions.
In this work we conclude that the paradigm of the greenhouse effect, by the absorption of infrared energy (heat) by the 0.04 per cent of the gases in the atmosphere, is the main source of heat of the planet should be reconsidered, because 99.9% of the gases in the atmosphere, do not absorb the heat. But this work showed that the absorption of the ultraviolet energy by the oxygen (21% of the gases in the atmosphere) and processes physicochemical originated (ionization), is the best way to explain the heat in the earth, Also the explanation of global warming, because the oxygen is percentage much more abundant than the greenhouse gases, to amount to 21 per cent of the entire atmosphere, and the characteristics of the oxygen, to be the only gas highly reactive in the atmosphere, which when it absorbs energy, higher energy level that the infrared, in this case the ultraviolet, not only accelerates the speed of the molecules of the 21% of the atmosphere, Increasing the temperature, but that it has the characteristic of cause heat and light, when it absorbs this energy, through processes physical chemicals known as ionization, processes that occur in all layers of the atmosphere, but that have been studied in the higher layers of the atmosphere, as in the stratosphere and the Ionosphere.
This process operates as follows in the atmosphere, the oxygen to absorb ultraviolet causes heat directly. Because it is ionized, which converts the oxygen in a conductor of electricity in the air, therefore heat, the Joule effect explains this, as an irreversible phenomenon through which, if a driver circulates electric current, part of the kinetic energy of the electrons is transformed into heat, which happens with the electricity in the air, That is why in the middle of the day that there is much more electricity in the air makes it more heat, which in the morning or in the evening where the electricity in the air is less, we can also conclude that the electricity in the air, is the product of the ionization of oxygen from the atmosphere.
This work also concludes that the increase of CO2 in the atmosphere, does not increase the average temperature of the planet, but that the heat on the planet if it can increase the amount of CO2 on the planet, because satellite images show us that in the warmer weather, less industrialized countries , and that burn less fossil fuel, can shed the planet more CO2 to the atmosphere, because heat increases the decomposition of the large reserves of vegetation found in these countries.
Another outcome of this work is that the greenhouse effect, that there are planets Venus, cannot explain the temperature or the planet Venus, nor in the earth. In addition, that the ionization of the atmospheres is the best explanation for the high temperatures, which we see in the layer of the atmosphere called the ionosphere, which reaches more than the 1500ºC temperatures, registering temperatures 3 times greater than the planets that are closer to the sun.
In conclusion, there is a reality that has been overlooked by science, is that in the troposphere there is the greatest amount of oxygen in the atmosphere and the ultraviolet energy reaches this layer causing, all physicochemical process that cause heat. In addition, the oxygen is used to increase the temperature of oven atmospheres, in smelting industries, and CO2 is used to decrease the temperature of these kilns.
After verifying that the heat on the planet is not mainly due to a greenhouse effect, but to the absorption of the ultraviolet energy by the oxygen of the atmosphere, we can understand the problematic of the climatic change, in a different way, since the Warming on the planet could not be global, because the greenhouse effect of the planet has already been ruled out, but that the warming of the planet, would depend on the places on the planet that receive the largest amount of ultraviolet rays, and where to concentrate the largest reserves of oxygen in the troposphere, depending on these two factors, the most necessary solutions should be sought.
Also, according to the percentage contained in the atmosphere of gases that originate heat, it is concluded that 99.8% of the heat on earth is caused by oxygen, and 0.2% is the amount of heat that emit greenhouse gases in the earth.
bibliography :
[1] http://www.rsc.org/images/Arrhenius1896_tcm18-173546.pdf ...
Página 267
[2] https://elpais.com/sociedad/2012/08/29/actualidad/1346264531_379503.html
[3] https://www.elconfidencial.com/tecnologia/2015-01-19/el-video-de-la-nasa-que-muestra-como-ha-aumentado-la-temperatura-en-la-tierra-durante-los-ultimos-135-anos_623966/
[5] https://es.wikipedia.org/wiki/Electricidad_atmosf%C3%A9rica
[6] https://es.wikipedia.org/wiki/Ionizaci%C3%B3n
[7] https://es.wikipedia.org/wiki/Mercurio_(planeta)
[10] https://es.wikipedia.org/wiki/Ionosfera
[11] Gillis, Justin (28 de noviembre de 2015). «Short Answers to Hard Questions About Climate Change». The New York Times. Consultado el 7 de marzo de 2016.
[12] Hartmann, D. L.; Klein Tank, A. M. G.; Rusticucci, M. (2013). FAQ 2.1 «2: Observations: Atmosphere and Surface» (PDF). IPCC WGI AR5 (Report) (en inglés). Evidence for a warming world comes from multiple independent climate indicators, from high up in the atmosphere to the depths of the oceans. They include changes in surface, atmospheric and oceanic temperatures; glaciers; snow cover; sea ice; sea level and atmospheric water vapour. Scientists from all over the world have independently verified this evidence many times.
«Myth vs Facts....» (en inglés). EPA (US). 2013.The U.S. Global Change Research Program, the National Academy of Sciences, and the Intergovernmental Panel on Climate Change (IPCC) have each independently concluded that warming of the climate system in recent decades is 'unequivocal'. This conclusion is not drawn from any one source of data but is based on multiple lines of evidence, including three worldwide temperature datasets showing nearly identical warming trends as well as numerous other independent indicators of global warming (e.g., rising sea levels, shrinking Arctic sea ice).
[13] Climate Change 2013: The Physical Science Basis - Summary for Policymakers, Observed Changes in the Climate System, p. 2 (en inglés), en IPCC AR5 WG1, 2013. «Warming of the climate system is unequivocal, and since the 1950s, many of the observed changes are unprecedented over decades to millennia.
[14] Costa, M. et al.. 2009. Ciències de la Terra i del Medi Ambient. Ed. Castellnou, Barcelona. ISBN 978-84-9804-640-3
[17] http://www.natureworldnews.com/articles/7957/20140708/depleting-ozone-may-lead-to-increased-ultraviolet-radiation-on-earth.htm
[18] http://www.sunsmart.com.au/about/media-campaigns/media-releases/2012-media-releases/media_release_20120916.html
https://es.wikipedia.org/wiki/Temperatura_atmosf%C3%A9rica
[23] https://www.carburos.com/industries/GlassMinerals/Oxy-Fuel-Techniques.aspx
[25] Costa, M. et al.. 2009. Ciències de la Terra i del Medi Ambient. Ed. Castellnou, Barcelona. ISBN 978-84-9804-640-3
[26] http://www.stat.yale.edu/~jah49/Pictures_in_R/Austria_Warming/AustriaWarming.pdf
[29] Source for figures: Carbon dioxide, NASA Earth Fact Sheet, (updated 2007.01). Methane, IPCC TAR table 6.1, (updated to 1998). The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2by about 7 ppmv.















Comentarios
Publicar un comentario